
1. Transition elements can form coloured compounds
2. Transition elements have varying oxidation number
- Iron has two common oxidation states (+2 and +3) in, for example, Fe2+ and Fe3+. It also has a less common +6 oxidation state in the ferrate(VI) ion, FeO42-.
- Manganese has a very wide range of oxidation states in its compounds. For example:
| +2 | in Mn2+ |
| +3 | in Mn2O3 |
| +4 | in MnO2 |
| +6 | in MnO42- |
| +7 | in MnO4- |
3. Transition elements can form complex ions
- A complex ion has a metal ion at its centre with a number of other molecules or ions surrounding it.
- Some examples of complex ions formed by transition metals
- [Fe(H2O)6]2+, [Co(NH3)6]2+, [Cr(OH)6]3- , [CuCl4]2-
4. Transition elements can act as catalysts
- Iron in the Haber Process
The Haber Process combines hydrogen and nitrogen to make ammonia using an iron catalyst.
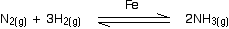
- Vanadium(V) oxide in the Contact Process
At the heart of the Contact Process is a reaction which converts sulphur dioxide into sulphur trioxide. Sulphur dioxide gas is passed together with air (as a source of oxygen) over a solid vanadium(V) oxide catalyst.

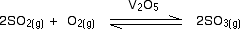
- Nickel in the hydrogenation of C=C bonds
This reaction is at the heart of the manufacture of margarine from vegetable oils.
However, the simplest example is the reaction between ethene and hydrogen in the presence of a nickel catalyst.


No comments:
Post a Comment